what is the procedure performed to gain access to the airway below a tracheal obstruction?
Review Article
Management of cancerous airway obstruction
Introduction
The term "Cancerous Cardinal Airway Obstruction" (MCAO) refers to any malignant, mechanical, obstructive process that impedes the airflow inside the central airways (trachea, main-stem bronchi, and right bronchus intermedius). MCAO represents a substantial source of morbidity and mortality with a significant impact on quality of life (ane,2).
Malignant obstruction of the central airways usually presents late in the course of the illness, and near individuals have a limited life expectancy. It creates pregnant physiological and psychological distress in patients and occasionally, may present as imminent arrest from suffocation. Traditional modalities of treatment, such as chemotherapy and external beam radiations are ineffective for the acute direction of severe MCAO, non simply in restoring oxygenation and ventilation, simply also in palliating symptoms. Multiple highly effective interventional pulmonology techniques are now bachelor for the management of malignant obstacle of the central airways.
Prevalence
The actual incidence and prevalence of MCAO are unknown, though an estimated fourscore,000 cases of malignant airway obstacle are treated annually in the U.s. (3). Unfortunately, information technology is non infrequently seen in clinical practice, specially in avant-garde malignancies. Approximately twenty% to 30% of patients with lung cancer may develop a complication related to fundamental airway obstruction, such every bit dyspnea, atelectasis, hypoxemia, hemoptysis, post-obstructive pneumonia or respiratory distress. Most 40% of lung cancer-related deaths result from complications due to advanced loco-regional disease (2,4-7).
Lung cancer remains the leading cause of cancer decease in both men and women in the U.s.. Given the contemporary epidemiology of lung cancer, the incidence and prevalence of malignant CAO may exist increasing due to the rising number of lung cancer patients developing complications related to endobronchial disease (2).
Etiology
Malignant obstruction of the central airways may develop due to a primary intraluminal malignancy, extension of an adjacent tumor with airway invasion, metastatic endo-luminal disease, or extrinsic pinch from a face-to-face malignant process (8). The most common cause of MCAO is direct extension and invasion from an adjacent tumor, often bronchogenic carcinoma (ii,9). Squamous cell carcinoma accounts for more than than half of the not-minor-jail cell lung cancer (NSCLC)-related MCAO (10,xi). Other tumors unremarkably associated with adjacent endobronchial invasion include esophageal, laryngeal, and thyroid malignancies.
Primary malignancies of the airways are rare. Squamous cell carcinoma and adenoid-cystic carcinoma business relationship for ii-thirds of all primary tracheal malignant tumors (12-16). Distal to the chief carina, carcinoid tumors account for many main airway malignancies, typically presenting every bit discrete central endobronchial lesions (16,17).
Endoluminal metastases from afar malignancies are relatively uncommon, with the reported incidence ranging widely from 2% to 50%. The considerable variation is likely related to the variable definitions used; the incidence is much lower when merely afar tumors that directly metastasize to the airways are considered (18,nineteen). In one autopsy series of patients with solid tumors, metastatic disease to the primal airways occurred in only ii% of the cases (twenty). Even so, the incidence of extra-thoracic endoluminal metastases presenting as symptomatic MCAO is non known. A wide variety of tumors that metastasize to the airways have been described, including chest, colorectal, renal jail cell, and thyroid carcinomas (2,xviii,xix).
Types/classification (Effigy 1)
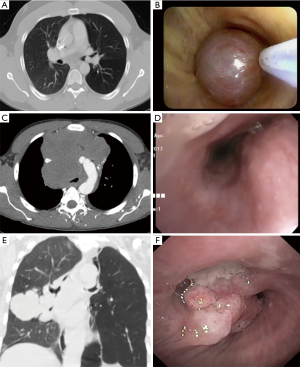
Effigy 1 Types of cancerous endobronchial obstruction: CT and bronchoscopic images. Intrinsic compression: (A) A pure endoluminal carcinoid tumor is seen at left principal stem; (B) the endobronchial carcinoid is removed by snare electrocautery. No external compression is seen. External compression: (C) large anterior mediastinal mass causing severe distal tracheal obstruction; (D) bronchoscopic image of severe distal tracheal compression and complete right primary stem apoplexy. Mixed: (Eastward) right middle lobe adenocarcinoma of the lung next to the bronchi with invasion into the airway and external compression; (F) endoluminal tumor with obstruction of the right eye lobe and bronchus intermedius compression.
Three basic types of MCAO have been widely described:
- Intrinsic or endoluminal obstruction: Airway lumen compromised purely by an endo-bronchial/tracheal obstructive tumor.
- Extrinsic or extraluminal obstruction: Airway compressed by an actress-bronchial/tracheal cancerous process.
- Mixed obstruction: A combination of intraluminal and extraluminal airway obstruction.
Clinical presentation
MCAO not only carries a very poor prognosis if left untreated, but too results in meaning daily life disturbances. Patients are often quite symptomatic with severely impaired quality of life. The symptomatology of MCAO is diverse and nonspecific, with shortness of breath and coughing beingness the most commonly reported symptoms. Other frequently reported symptoms include hemoptysis, hoarseness, chest discomfort, orthopnea, and dysphagia (21). Patients may be asymptomatic if the obstruction is mild. Conversely, even in mild obstructions during an acute respiratory infection, the concomitant edema and buildup of secretions may decrease the airway lumen at the obstructive site, leading to meaning symptoms. Shortness of breath is usually a late sign that may develop at residual or on exertion. Information technology is occasionally positional with dyspnea occurring in the supine position due to airway compression by large intrathoracic tumors. Dyspnea characteristically is persistent and not responsive to bronchodilators. The level of shortness of jiff does not e'er correlate with the amount of airway obstruction. Cough is ordinarily chronic, persistent, and may be dry out or productive of purulent sputum. Hemoptysis is common. Though nearly studies written report a mild to moderate caste, massive hemoptysis may occur. Dysphagia may exist nowadays in patients with large airway malignancies causing esophageal pinch, or with esophageal malignancies with endobronchial invasion (2,22,23).
On chest auscultation, stridor, wheezing, localized crackles, or frank consolidation may exist encountered, depending on the location and size of the obstacle-related atelectasis. The location of wheezing does not ever follow the site of the airflow obstacle and may be heard over the trachea or lung fields (24). Unilateral wheezing suggests obstruction distal to the carina. Wheezing related to MCAO is mostly non responsive to bronchodilators (23,25).
Piece of work-up/imaging/diagnostic bronchoscopy
The diagnosis of MCAO tin be challenging. It requires a high clinical suspicion and is normally based on a combination of past medical historic factors and feature findings on physical examination, also as physiologic, imaging, and endoscopic studies. A detailed clinical history is crucial for diagnosis. The past medical history must be advisedly reviewed, and previous medical records obtained (26). Any agile or previous cancerous diseases should be thoroughly researched, as most patients with MCAO have an end-stage main tumor or a recurrence post-obit prior surgical or chemo-radiation regimens (27). A family history of malignancy should besides be too noted.
A plain breast X-ray should be obtained in every patient suspected to have MCAO. Although it is infrequently diagnostic and has a low sensitivity detecting abnormalities of the trachea and main bronchi, it may reveal obvious pathologies such as tracheal departure from an side by side lesion, lobar atelectasis, or complete lung collapse (28,29).
Computed tomography (CT) of the thorax is the well-nigh accurate noninvasive method of airway evaluation, allowing diagnosis and handling planning in MCAO. CT as an imaging modality is superior to breast 10-ray in detecting tracheal and main bronchi abnormalities, with a reported sensitivity of 97% (28). A CT of the breast helps determine the type of obstructive lesion (intraluminal, extrinsic, or mixed), the patency of the airway distal to the obstruction, and of import lesion characteristics, including length, diameter, and human relationship to nearby structures such as vessels. Intratracheal or intrabronchial masses and signs of extrinsic tracheal or bronchi pinch may be evident (Figure two) (30,31). Post-obstructive pneumonia, atelectasis, and lobar collapses may also exist visible. CT of the chest with multi-planar reconstructions has a reported 93% sensitivity, 100% specificity, and 94% accuracy in demonstrating the site and caste of tracheal and main bronchi stenosis (32).
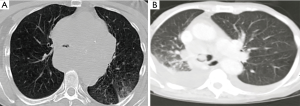
Figure ii Chest CT images demonstrating airway obstacle from extrinsic and intrinsic tumors. (A) Big mediastinal mass causing severe obstruction of the trachea; (B) large endobronchial mass (arrow) at the master carina.
Bronchoscopy, either flexible or rigid, is the near specific and sensitive test for MCAO and is used for the assessment, diagnosis and treatment, frequently simultaneously during the initial process (33,34). Directly visualization via bronchoscopy tin provide information on the location and morphology of the lesion, the amount of intraluminal disease, the presence of extraluminal compression, and the diameter and length of the lesion. It as well allows evaluation of the surrounding tissue, particularly the airway distal to the obstacle. Additionally, tissue tin can exist obtained for pathologic diagnosis and staging if necessary. Diagnostic flexible bronchoscopy has several detriments and should be performed with circumspection. It does not provide a complete evaluation of extraluminal disease or the viability of the airways distal to the obstruction. It may also be difficult and potentially dangerous in severe obstruction, as the bronchoscope itself volition farther obstruct the already narrow lumen, particularly if secretions, edema, or haemorrhage occur (27). The moderate sedation required during the procedure may decrease ventilation and relax the respiratory muscles, creating a potentially unstable airway, particularly with anterior mediastinal tumors. For these reasons, an interventional pulmonology team is crucial when diagnostic flexible bronchoscopy is performed in MCAO. In cases of severe obstruction presenting with impending respiratory failure, diagnostic studies including preliminary flexible bronchoscopy may not exist possible, and a rigid bronchoscopy should exist performed immediately.
Management
The management of MCAO is challenging and requires a multidisciplinary team approach with the involvement of a pulmonologist, medical and radiation oncologist, anesthesiologist, ENT specialist, thoracic surgeon, and interventional pulmonologist. Surgery is oftentimes non indicated due to the advanced illness country or the patient'due south comorbidities. Chemotherapy has inconsistent and delayed beneficial furnishings and radiotherapy often yields suboptimal results, with delayed atelectasis resolution obtained in only half of the cases (35,36).
Management is more often than not dependent on the initial presentation. Approximately forty% of the interventions performed for airway obstacle are done either on an urgent or emergent basis (37). Significant cancerous airway obstruction presenting with severe respiratory distress requires firsthand action to promptly and finer re-establish and secure the airway too as to relieve the obstacle (27). Thus, in unstable patients presenting with severe tracheal or bronchial obstruction and impending respiratory failure, initial stabilization should exist focused on establishing a secure airway. This may crave endotracheal intubation or urgent rigid bronchoscopy. If an interventional pulmonology squad is not available, patient transfer to a specialized center should be considered after initial stabilization.
Malignant airway obstruction often presents in a late stage where curative surgical resection is not an option due to the extent of malignant illness at presentation and the existence of comorbid medical weather condition that render many patients unsuitable for surgery (34,38). Nonetheless, when feasible, resection of the malignant procedure is the treatment of choice. Retrospective studies suggest that therapeutic bronchoscopy can be used as a complementary tool in the management of MCAO prior to curative surgery (xi).
Additionally, few cases have described not-resectable lung cancers that accept become operable post-obit interventional bronchoscopic therapies (x,39). Unfortunately, the majority of lung cancer patients present in avant-garde stages, either stage III or stage 4 (40), therefore management is focused primarily on symptom palliation and quality of life improvement.
In patients with inoperable tumors of the central airway, restoration of airway patency provides palliation and may prolong life, peculiarly in cases presenting with impending respiratory failure (33). No established guideline exists for the management of MCAO. Several techniques are available for relieving the airway obstacle; the choice of which to use depends on the obstruction type, the patient's clinical condition, equipment availability and treating physician's expertise. Interventional bronchoscopy procedures may exist indicated prior to chemotherapy or radiation therapy (or when such treatment fails) and have been shown to improve dyspnea equally well as mechanical ventilation liberation rates, thus increasing quality of life (10). Consequently, the 2013 American College of Chest Physicians (ACCP) evidence-based clinical exercise guidelines recommend that in patients with inoperable lung cancer and symptomatic airway obstacle, therapeutic bronchoscopy with mechanical or thermal ablation, brachytherapy, or stent placement, should be offered with the aim of improving dyspnea, cough, hemoptysis, and quality of life (41).
Endoscopic direction
Multiple and often complementary techniques exist in the armamentarium of interventional pulmonology for the direction of MCAO. Selection of the advisable approach depends on several factors, including the vigil of the presentation, the underlying crusade, the type of lesion, the stability of the patient, the patient'due south general, cardiac, and pulmonary status, the quality of life, the overall prognosis, the dr. expertise, and the technology available (42-44). Multimodal direction utilizing various endoscopic techniques is by and large used (Figure 3) (45).
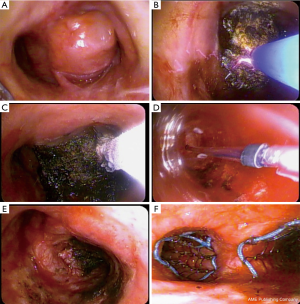
Figure 3 Multimodal management of MCAO: (A) Large endobronchial lesion causing near complete obstruction of the right main stem and external pinch of the left principal stem; (B) argon plasma coagulation in use for tumor carbonization; (C) cryotherapy probe in use for debulking by cryo-adhesion; (D) balloon bronchoplasty for dilatation prior to stenting; (E) right main stem afterwards recanalization; (F) bilateral partially covered self-expanding metal stent placement.
Subsequently, we will draw the more than frequently used procedures.
Rigid and flexible bronchoscopy
Bronchoscopic therapy, performed via flexible or rigid bronchoscopy, results in an improvement in symptoms, quality of life, and survival. Both techniques may be complementary. Rigid bronchoscopy offers first-class airway control. It is a safe and highly effective way of securing the airway while providing the capability to ventilate and oxygenate during diagnostic and therapeutic airway procedures (34,45-47). Rigid bronchoscopy is considered by experts the modality of selection in patients with impending respiratory failure. It requires full general anesthesia and an operating theater. Contraindications include those related to anesthesia every bit well every bit circuitous anatomy of the neck and mandible (i.eastward., unstable cervical spine, oral or maxillofacial trauma) (46). Complications are infrequent with the most common being a sore throat afterwards the procedure. Other reported complications include injury to the teeth, gums or lips, tracheal or bronchial tears, severe bleeding, and hypoxemia-induced cardiac ischemia and arrhythmia. The overall mortality related to rigid bronchoscopy is as low as 0.four% (45-48). Over the past several years, various improvements have occurred to the design of the rigid bronchoscope, including the ability to measure out inspiratory and expiratory pressures too every bit oxygen and carbon dioxide concentrations, creating a more than versatile tool (Figure 4) (49,fifty). Rigid bronchoscopy requires specialized training and as such, is underutilized in the United States due to the scarcity of preparation environments. Rigid bronchoscopy training is offered in iv.4% of all pulmonary medicine programs and simply 31.iii% of pulmonary programs with an interventional pulmonology service (48).
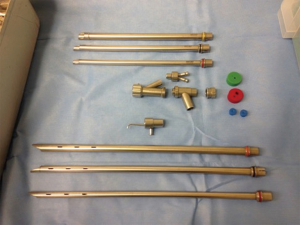
Figure 4 A "Bryan-Dumon" rigid tracheoscope and bronchoscope. Note the length of the tracheal scopes and the bronchial scopes (with orifices for ventilation).
Thermal techniques
All thermal endoscopic airway interventions can exist used with either a rigid or a flexible bronchoscope.
Light amplification of stimulated emission of radiation (LASER)
Laser photoresection refers to the application of Laser energy to produce thermal, photodynamic, and electromagnetic changes in tissues. Numerous types of LASER are available, including neodymium: yttrium-aluminum-garnet (Nd:YAG), carbon dioxide (CO2), and neodymium: yttrium-aluminum-perovskite (Nd:YAP), amid others. Nd:YAG LASER is probably the well-nigh widely used for endobronchial illness. Nd:YAG is a noncontact or contact technique in which nosotros apply thermal energy to the airway tissue for the relief of cancerous obstruction. The Laser thermal technique can be used in emergent situations and is an excellent musical instrument for rapid endoluminal debulking, with a reported charge per unit of 83% to 93% of lumen re-establishment and a 63% to 94% rate of symptom palliation. Excellent cognition of anatomy is essential to avoid complications, such equally major vessel perforation. As the depth of tissue destruction cannot be precisely assessed just past the advent of the tissue surface, extreme caution should be taken to direct the laser beam parallel to the bronchial wall to avoid damage. To minimize movement, general anesthesia with neuromuscular relaxant is recommended (due east.g., cough). Light amplification by stimulated emission of radiation, every bit with other thermal techniques, is not indicated in isolated extrinsic MCAO. Complications are rare in experienced hands and are estimated to be <3%. Such complications include perforation (of airways, esophagus, or vessels), cardiac arrhythmias, pneumothorax, hemorrhage, hypoxemia, myocardial infarction, air embolism (secondary to gas leaving the probe tip under pressure and crossing the mucosal membranes into the blood vessels), and endobronchial ignition. The fraction of inspired oxygen (FiO2) should remain less than twoscore% during the procedure to avoid endobronchial fire. Currently, no randomized trials exist to compare Nd:YAG LASER with other forms of MCAO management (i.e., electrocautery or cryotherapy), but several retrospective studies have shown successful outcomes using Nd:YAG Laser by itself or in combined modality therapy (2,nine,26,27,43,45,47,51-56).
Electrocautery
Electrocautery or electrosurgery refers to the use of an electrical electric current to heat and destroy tissue. Information technology can exist a contact or noncontact technique in which a high-frequency alternating electric current is delivered to the obstructive tissue for the relief of a malignant obstacle. The effect on the tissue depends on the power used, the application fourth dimension, the contact surface surface area, and the tissue blazon. The heat generated past the electric current is proportionally related to tissue resistance, and inversely related to tissue vascularity and moisture content. Depending on the blazon of accessory apply, the Power (W) settings range from 10–xl West. Electrocautery may be used in emergent situations and is an excellent tool for rapid endoluminal debulking, with a reported rate of lumen re-institution of approximately >xc%, and symptomatic improvement in more than than 90% of patients. A diverseness of tools exist to use electrocautery, via either rigid or flexible bronchoscopy. For the flexible bronchoscope, electrocautery snares, knives, blunt probes, and hot forceps are bachelor. Electrocautery should be avoided in pure extrinsic airway compression. In patients with pacemakers or automated implantable cardioverter/defibrillators (AICDs), due to the potential for dysrhythmias or device malfunction, caution is recommended, and the device should be turned off whenever possible and clinically indicated. Complications are rare, estimated between 2–5%, and include hemorrhage, endobronchial ignition, electric shock to the operator, and airway perforation. Loss of efficacy tin occur with haemorrhage due to the diffusion of the current beyond a larger surface area. The FiO2 must be below 40% to avoid an airway fire (2,42,43,47,54,56-59).
Argon plasma coagulation (APC)
APC is a non-contact mode of tissue electrocoagulation in which ionized argon gas is used to conduct electrical electric current to the obstructive tissue for the management of MCAO. APC is used more than frequently as an alternative to Light amplification by stimulated emission of radiation therapy and electrosurgery, every bit information technology is an excellent tool for photocoagulation (hemostasis) with a charge per unit of luminal restoration of approximately 91% and a remarkable safe profile. Iii types of flexible APC probes are bachelor for different indications: 0-degree or head-on, radial and lateral. APC tin can target lateral lesions or those at sharp angles to the probe. APC can be used in a forced or pulse mode, and the power setting may be fix between 10–thirty W. Even so, APC does not crusade tumor vaporization, thus requiring other modalities for the debulking of large tumors. After applying APC to an endoluminal tumor, the resultant eschar and debris must be removed with suction, forceps, or cryoadhesion. As with LASER and electrocautery, APC is contraindicated in extrinsic airway compression, and the aforementioned principle of caution applies for patients with pacemakers or AICDs. A complication rate of <i% has been reported. Complications include hemorrhage, airway perforation, airway stenosis, endobronchial ignition, and air embolism from the argon gas. The recommended menses of gas is from 0.3 to 0.viii LPM to avoid an embolism upshot. Also, every bit with the previously described thermal modalities, the FiO2 must be below 40% to avert an airway fire (2,43,47,54-56,59,sixty).
Cryotherapy
Cryotherapy utilizes farthermost common cold to destroy tissue past rapid freezing and slow thawing cycles. Cryotherapy for endobronchial tumor management was first described in 1968 (61). Information technology is a contact technique with cryogen (i.e., nitric oxide) application to the obstructive tissue. Most of the effects of cryotherapy outset several hours after treatment, therefore historically the use of cryotherapy in malignant airway obstructions was express to non-acute obstruction.
Information technology is also important to remember that tissue necrosis and sloughing at 1–2 weeks, requires removal of debris to achieve the desired event (43,45,56,62,63). Nevertheless, with the use of cryoadhesion, debulking, and recanalization, the role of the cryoprobe in malignant endoluminal airway obstacle has expanded. Case reports (64) and retrospective studies take shown that the use of cryotherapy for cryorecanalization can achieve an immediate handling outcome with an adequate prophylactic and efficacy profile (61). A large retrospective study of 225 cases found a 91% success rate using the flexible cryoprobe for cryo-recanalization of malignant stenosis (65). Cryotherapy is a safe procedure with few and relatively small complications. In the published literature, the most important complexity of cryorecanalization is bleeding. Experienced users of cryo-debridement recommend the use of other techniques for hemostasis (i.due east., APC or electrocautery) in as many as 8% to x% of cases (61,65-67). One of the principal advantages of using cryotherapy up front in MCAO debulking is the ability to diagnose (cryobiopsies) and debulk with recanalization equally the initial diagnostic and therapeutic process.
Not-thermal techniques
Mechanical debulking by rigid bronchoscopy
The employ of isolated mechanical debulking for treatment of MCAO has been previously described.
A retrospective study has shown that rigid bronchoscopy and mechanical debulking every bit a sole therapy is safe and successful in up to 83% of cases of central airway tumors (68). In rigid debulking, the distal end of the rigid tracheoscope or bronchoscope acts equally a corkscrew for dilating the stenotic segment, or every bit an apple corer penetrating through large obstructive tumors. The butt of the bronchoscope can be used simultaneously to tamponade haemorrhage lesions while debulking the tumor. Large forceps may be introduced through the bronchoscope to assist in the mechanical debridement of large tumors. A flexible bronchoscope may exist used during rigid bronchoscopy to facilitate tissue debridement in angulated or distal airways. These techniques, although still commonly used, should be reserved for the most astringent cases. Complications with mechanical debulking range from ane–20%, and include pneumothorax, hemoptysis, and pneumonia (69).
Airway dilation
Dilation of airway obstructions due to intrinsic, extrinsic or mixed MCAO may be achieved with insertion of the butt of a rigid bronchoscope or with balloon dilation. Rigid bronchoscopic airway dilation can be used in emergent situations, as rapid recanalization can be achieved every bit described above (33,46). Balloon dilatation or bronchoplasty (BBP) tin can exist performed during rigid or flexible bronchoscopy with or without fluoroscopy and involves the use of increasingly larger diameter balloons filled with saline maintained in position for 15–60 s to amplify the airway. This induces less mucosal trauma and subsequent granulation tissue germination than rigid dilation. BBP results in an firsthand improvement in extrinsic and intrinsic MCAO in upwards to 79% of patients and oftentimes used for airway dilation before stenting. As its furnishings are non long-lasting, BBP dilation is frequently combined with other therapies such as light amplification by stimulated emission of radiation resection, radiation therapy, or stenting. Complications include stenosis recurrence, pain, mediastinitis, and haemorrhage, too as airway fierce or rupture with subsequent pneumothorax or pneumomediastinum (70,71).
Microdebrider
The microdebrider is a powered rotating blade utilise to resect endoluminal obstructions. Simultaneous suction facilitates rapid removal of blood and debris with minimal trauma to the airway. The microdebrider blade may exist smooth or serrated and comes in ii lengths: 37 cm, which is used to admission lesions of the trachea and the well-nigh proximal primary bronchi, and 45 cm, used for more distal lesions. The usual speed of the blade is between 1,000 and ii,000 rpm. The microdebrider represents an alternative therapeutic option in patients with poor pulmonary reserve as at that place is no demand to decrease the FiO2 during tumor debulking. Additional techniques such equally electrocautery may be necessary to achieve hemostasis in up to 35% of patients. Retrospective studies have found that the microdebrider is condom and effective in the direction of MCAO (72-74).
Airway stents
Airway stents are prostheses of diverse materials used to support and maintain patency of the airway. Two types of stents (silicone and metallic/hybrid) are oftentimes used in the direction of MCAO. Airway stents are best suited for extrinsic cancerous compression. They are sometimes used to maintain airway patency after intrinsic or mixed endobronchial tumor ablation, or in cases of persistent airway narrowing. Stents deliver firsthand and durable palliation, with symptomatic relief accomplished in up to 84% of patients. Tracheobronchial stents improve quality of life and survival in patients with avant-garde malignant obstruction. When used for prolonged periods of time, stents may develop significant complications. Reported complications include migration, infection, granulation tissue formation, halitosis, stent fracture, metallic fatigue, perforation of vessels and airway wall, mucosal tears, and obstruction of lobar orifices. Experts recommend a "stent alert" carte to exist given to every patient. It should specify the type and size of the stent, the location, and the appropriate size of the endotracheal tube to exist used if emergency intubation became necessary.
Silicone stents (i.e., Dumon bronchial stent, Dumon-Y stent, Polyflex stent) are placed under rigid bronchoscopy and have several benefits including varying caste of compactness and flexibility to simultaneously mold to the airway while resisting external compression. They are relatively inexpensive and are reasonably well-tolerated (75). Worldwide silicone stents are the most commonly used stents (40). The Dumon silicone stent has studs on the surface to decrease the migration rate. They can exist customized to the patient needs prior to insertion (i.e., cut to the desired length, create an orifice for a lobar bronchus) I meaning advantage of the silicone stents is the fact that they tin can be removed with ease. The Dumon-Y silicone stent is particularly useful in malignant diseases involving the carina and the mainstem bronchi. They do, all the same, take loftier rates of migration and obstacle as granulation tissue forms at the stent-ends and mucous secretion clearance is impaired (Figure 5).
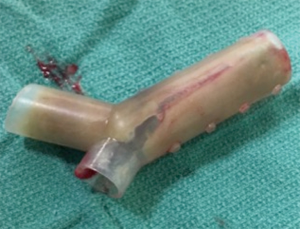
Effigy 5 A Dumon-Y stent with about consummate apoplexy due to mucous plugs secondary to impaired fungus secretions clearance.
Self-expanding metallic stents are relatively easy to deploy without the need of rigid bronchoscopy and arrange improve to disrupted airways. They have improve internal-to external bore ratios and therefore result in larger airway lumens overall. Unfortunately, tumor and granulation tissue often grow through the mesh, making purely metal stents challenging to remove. Complications such every bit airway and vessel perforation have been reported (Effigy 6) (2,6,43,54,75-81).

Figure 6 Note the self-expanding metallic stent placed at the right lower lobe in contact with the pulmonary avenue branch. The patient afterward developed massive hemoptysis.
Hybrid stents (i.e., Ultraflex stent, Aero stent), in theory, combine the qualities of silicone and metal stents. The covered hybrid stents have the reward of creating a mechanical barrier, preventing tumor ingrowth and are in general easier to manipulate. Some of these stents take a pocket-size proximal or distal loop allowing for partial collapse and subsequent removal or reposition. Others have anti-migration struts which sink into the mucosa to prevent migration, causing pregnant mucosal injury, particularly at the time of removal (75).
Photodynamic therapy (PDT)
In PDT, a light of specific wavelength [from a potassium titanyl phosphate (KTP) laser] is practical to the obstructive lesion via a flexible bronchoscope 48 to 72 hours after the systemic injection of a photosensitizing drug such every bit dihematoporphyrin ester (DHE). This leads to a phototoxic reaction and tumor destruction as the photosensitizing drug is preferentially taken up by cancerous cells. Immediately and up to 48 hours after the procedure, bronchoscopic toilet (cleaning and debulking of the surface area to remove tumor debris, retained secretions, and sloughed mucosa) is performed to found airway patency and assess the necessity of farther treatment. PDT is indicated in the palliative treatment of CAO without acute dyspnea and is particularly useful in distal obstructions due to cancerous endobronchial masses with minimal extrinsic airway compression. Due to the delayed response of treatment, PDT should not exist used in the emergent management of acute, severe MCAO. The about common complication associated with PDT is skin photosensitivity. Patients receiving PDT should be advised to avert sun exposure for 4–6 weeks after the procedure. Other complications include local airway edema, strictures, hemorrhage, and fistulae formation, although PDT has a low risk of airway perforation. The furnishings of PDT are relatively long-lasting and have been shown to palliate airway obstruction in fourscore% of patients (2,42,43,47,54).
Radiation (brachytherapy) endoscopic airway interventions
Endobronchial delivery of radiations is accomplished via the placement of a radioactive substance (i.eastward., iridium-192) directly into or near the airway tumor using a flexible bronchoscope. This results in tissue destruction through Dna mutations leading to prison cell apoptosis. Brachytherapy is indicated in the palliation of symptoms (particularly dyspnea, coughing, and hemoptysis) related to airway obstruction. High-dose endobronchial brachytherapy is too successful in the handling of excessive granulation tissue formation as a complication of airway stenting. As brachytherapy takes up to 3 weeks to be effective, information technology should non be used in the emergent management of acute, severe MCAO. The effects of brachytherapy are long-lasting, with a reported rate of lumen restoration of 78% to 85% and symptom relief of 69% to 93%. An advantage of brachytherapy is that it can be used for tumors in areas not accessible to other handling modalities (e.g., the upper lobe bronchi and segmental bronchi). Brachytherapy is delivered via low-dose-charge per unit (LDR) or high-dose-rate (HDR) endobronchial methods. High-dose endobronchial brachytherapy delivers higher radiation doses with less fourth dimension per fraction, thus permitting outpatient therapy. Brachytherapy can be used in combination with other techniques such as thermal therapy or external beam radiation, with which it has synergistic effects. Complications of brachytherapy include hemorrhage, mediastinal fistula formation, arrhythmias, hypotension, bronchospasm, bronchial stenosis or necrosis, and radiations bronchitis (45,47,54,63).
Outcome/prognosis
In the vast majority of cases, malignant airway obstruction is not curable, and the approach is aimed at the palliation of symptoms (due east.g., dyspnea, cough, hemoptysis). Patients and family members should be well aware of the palliative nature of these efforts. The survival of patients with untreated malignant CAO is mostly poor and ranges from i to 2 months (82). Furthermore, their quality of life is extremely poor, and they may dice with asphyxia or on mechanical ventilation. Several adventure factors for decreased survival in cases of MCAO accept been described, including a high American Society of Anesthesiologists (ASA) score, non-squamous cell histology, and previously untreated metastatic tumors (36). In patients with malignant airway obstacle, studies have shown that multimodal therapy and stent insertion improves quality of life when compared with other approaches.
A prospective study on patients with MCAO due to advanced or recurrent lung cancer who underwent LASER ablation demonstrated significant improvement in both objective and subjective measures of quality of life (83). Two retrospective studies showed improved palliation and improved survival subsequently airway stenting in advanced lung cancer (79,82). Additionally, a contempo prospective study showed that therapeutic bronchoscopy for MCAO significantly improved spirometry values (i.east., FVC, FEV1), quality of life scores and overall survival (84). Chhajed et al. (6) demonstrated that patients presenting with MCAO who had received interventional bronchoscopic therapy prior to oncological treatment, had the aforementioned expected survival as those patients presenting in the like stage without MCAO.
A recent report from the ACCP multicenter registry report of therapeutic bronchoscopy for MCAO showed a very loftier technical success rate of more than 90%. The highest success rates were associated with stent placement and endobronchial obstruction. A 48% clinical improvement in dyspnea was reported, with 42% improvement in health-related quality of life scores. A more meaningful improvement was seen in those patients who had greater dyspnea at baseline (85).
An overall complication rate of 3.ix% (range, 0.ix–11.seven%) was seen after therapeutic bronchoscopy for MCAO. The risk factors identified for complications included emergent/urgent procedures, an ASA score >three, redo-therapeutic bronchoscopy and moderate sedation. A fourteen.8% 30-solar day mortality was described (86).
Conclusions
In summary, MCAO is an important affliction entity which significantly impacts a patient's quality of life and can decide candidacy for systemic or surgical therapies. There are many minimally invasive bronchoscopic interventions which can be used to relieve MCAO, resulting in rapid relief of symptoms, even in acutely ill patients. Current modalities include a diverseness of thermal techniques, cryotherapy, mechanical debulking, airway dilation, and airway stent placement. Delayed therapies such as brachytherapy and photodynamic therapy are very useful in select cases. Thorough working knowledge of the risks and benefits of each modality is critical when individualizing a patient's handling program. A squad of experts including interventional pulmonologists and thoracic surgeons should exist involved in these cases.
Acknowledgements
Funding: None.
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series "Management of Complex Airway and Pleural Diseases". The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure grade (available at http://dx.doi.org/10.21037/amj.2018.11.06). The series "Management of Circuitous Airway and Pleural Diseases" was commissioned past the editorial function without whatsoever funding or sponsorship. Dr. Folch served as the unpaid Guest Editor of the series. The authors take no other conflicts of interest to declare.
Upstanding Argument: The authors are accountable for all aspects of the work in ensuring that questions related to the accurateness or integrity of any part of the work are appropriately investigated and resolved.
Open Access Argument: This is an Open Access article distributed in accordance with the Artistic Commons Attribution-NonCommercial-NoDerivs iv.0 International License (CC By-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are fabricated and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/iv.0/.
References
- Rafanan AL, Mehta AC. Part of bronchoscopy in lung cancer. Semin Respir Crit Intendance Med 2000;21:405-20. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker Hard disk drive, et al. Central airway obstacle. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Ernst A, Herth FJ. Principles and Practice of Interventional Pulmonology. S.l.: Springer-Verlag New York; 2013.
- Dutau H, Toutblanc B, Lamb C, et al. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest 2004;126:951-8. [Crossref] [PubMed]
- Miyazawa T, Miyazu Y, Iwamoto Y, et al. Stenting at the period-limiting segment in tracheobronchial stenosis due to lung cancer. Am J Respir Crit Care Med 2004;169:1096-102. [Crossref] [PubMed]
- Chhajed PN, Baty F, Pless Thou, et al. Upshot of treated advanced not-small cell lung cancer with and without cardinal airway obstruction. Chest 2006;130:1803-7. [Crossref] [PubMed]
- Guibert N, Mazieres J, Marquette CH, et al. Integration of interventional bronchoscopy in the management of lung cancer. Eur Respir Rev 2015;24:378-91. [Crossref] [PubMed]
- Davis MP. Supportive oncology. Philadelphia, PA: Elsevier/Saunders, 2011.
- Beamis JF Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med 2005;eleven:292-v. [Crossref] [PubMed]
- Cosano Povedano A, Muñoz Cabrera L, Cosano Povedano FJ, et al. Endoscopic treatment of central airway stenosis: five years' experience. Curvation Bronconeumol 2005;41:322-7. [PubMed]
- Chhajed PN, Eberhardt R, Dienemann H, et al. Therapeutic bronchoscopy interventions before surgical resection of lung cancer. Ann Thorac Surg 2006;81:1839-43. [Crossref] [PubMed]
- Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: xxx-two-twelvemonth experience. J Thorac Cardiovasc Surg 1996;112:1522-31. [Crossref] [PubMed]
- Mathisen DJ. Primary tracheal tumor management. Surg Oncol Clin Northward Am 1999;8:307. [Crossref] [PubMed]
- Webb BD, Walsh GL, Roberts DB, et al. Primary tracheal cancerous neoplasms: the Academy of Texas Medico Anderson Cancer Center experience. J Am Coll Surg 2006;202:237-46. [Crossref] [PubMed]
- El Marjany M, Arsalane A, Sifat H, et al. Chief adenoid cystic carcinoma of the trachea: a study of two cases and literature review. Pan Afr Med J 2014;19:32. [Crossref] [PubMed]
- Stevic R, Milenkovic B. Tracheobronchial tumors. J Thorac Dis 2016;viii:3401-13. [Crossref] [PubMed]
- Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg 2010;89:998-1005. [Crossref] [PubMed]
- Shepherd MP. Endobronchial metastatic affliction. Thorax 1982;37:362-5. [Crossref] [PubMed]
- Kiryu T, Hoshi H, Matsui Due east. Endotracheal/endobronchial metastases: clinicopathologic study with special reference to developmental modes. Chest 2001;119:768-75. [Crossref] [PubMed]
- Blasco M, Quadrelli SA, Bosio G, et al. Synovial Sarcoma and Endobronchial Invasion. J Bronchology Interv Pulmonol 2008;15:167-9.
- Bilaçeroğlu S. Endobronchial Ablative Therapies. Clin Chest Med 2018;39:139-48. [Crossref] [PubMed]
- Israel RH, Poe RH. Hemoptysis. Clin Chest Med 1987;eight:197-205. [PubMed]
- Jabbardarjani H, Herth F, Kiani A. Central Airway Obstruction Masquerading every bit Hard-to-Treat Asthma: A Retrospective Study. J Bronchology Interv Pulmonol 2009;16:half-dozen-9. [Crossref] [PubMed]
- Hollingsworth HM. Wheezing and stridor. Clin Chest Med 1987;8:231-twoscore. [PubMed]
- Mehta AC, Harris RJ, De Boer GE. Endoscopic management of benign airway stenosis. Clin Breast Med 1995;sixteen:401-13. [PubMed]
- Brodsky JB. Bronchoscopic procedures for primal airway obstruction. J Cardiothorac Vasc Anesth 2003;17:638-46. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Collins J, Stern EJ. Chest Radiology: The Essentials. Wolters Kluwer Health: Philadelphia 2007.
- Santacruz JF, Mehta Air conditioning. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;half dozen:79-93. [Crossref] [PubMed]
- Boiselle PM. Imaging of the large airways. Clin Chest Med 2008;29:181-93. [Crossref] [PubMed]
- Chung JH, Kanne JP. Multidetector-row Computed Tomography of Diffuse Tracheal Illness: Pictorial Review. J Bronchology Interv Pulmonol 2009;16:28-36. [Crossref] [PubMed]
- Whyte RI, Quint LE, Kazerooni EA, et al. Helical computed tomography for the evaluation of tracheal stenosis. Ann Thorac Surg 1995;lx:27-xxx. [Crossref] [PubMed]
- Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med 2001;344:740-nine. [Crossref] [PubMed]
- Jeon K, Kim H, Yu CM, et al. Rigid bronchoscopic intervention in patients with respiratory failure acquired by cancerous central airway obstruction. J Thorac Oncol 2006;1:319-23. [Crossref] [PubMed]
- Nihei 1000, Ishikura S, Kawashima M, et al. Brusque-course palliative radiotherapy for airway stenosis in non-small cell lung cancer. Int J Clin Oncol 2002;7:284-eight. [PubMed]
- Guibert Northward, Mazieres J, Lepage B, et al. Prognostic factors associated with interventional bronchoscopy in lung cancer. Ann Thorac Surg 2014;97:253-9. [Crossref] [PubMed]
- Ernst A, Simoff M, Ost D, et al. Prospective take a chance-adjusted morbidity and mortality issue analysis afterward therapeutic bronchoscopic procedures: results of a multi-institutional outcomes database. Breast 2008;134:514-9. [Crossref] [PubMed]
- Husain SA, Finch D, Ahmed One thousand, et al. Long-term follow-upwardly of ultraflex metallic stents in benign and malignant cardinal airway obstruction. Ann Thorac Surg 2007;83:1251-six. [Crossref] [PubMed]
- Sergio C, Foccoli P, Toninelli C, et al. Nd:YAG Light amplification by stimulated emission of radiation Therapy in Lung Cancer: An 11-Year Experience with 2,253 Applications in 1,585 Patients. J Bronchology Interv Pulmonol 1994;one:105-eleven.
- Ali MS, Sorathia L. Palliative Care and Interventional Pulmonology. Clin Breast Med. 2018;39:57-64. [Crossref] [PubMed]
- Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians testify-based clinical exercise guidelines. Breast 2013;143:e455S-e497S.
- Lee P, Kupeli E, Mehta Air-conditioning. Therapeutic bronchoscopy in lung cancer. Light amplification by stimulated emission of radiation therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med 2002;23:241-56. [Crossref] [PubMed]
- Folch E, Mehta Ac. Airway interventions in the tracheobronchial tree. Semin Respir Crit Intendance Med 2008;29:441-52. [Crossref] [PubMed]
- Du Rand IA, Hairdresser PV, Goldring J, et al. Summary of the British Thoracic Society guidelines for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:1014-5. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Club. Eur Respir J 2002;nineteen:356-73. [PubMed]
- Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med 2001;22:355-64. [Crossref] [PubMed]
- Ernst A, Silvestri GA, Johnstone D, et al. Interventional pulmonary procedures: Guidelines from the American Higher of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Alraiyes AH, Machuzak MS. Rigid bronchoscopy. Semin Respir Crit Care Med 2014;35:671-80. [Crossref] [PubMed]
- Yarmus L, Feller-Kopman D. Bronchoscopes of the twenty-kickoff century. Clin Chest Med 2010;31:nineteen-27. [Crossref] [PubMed]
- Dutau H, Vandemoortele T, Breen DP. Rigid bronchoscopy. Clin Chest Med 2013;34:427-35. [Crossref] [PubMed]
- Dumon JF, Reboud Eastward, Garbe 50, et al. Treatment of tracheobronchial lesions by laser photoresection. Breast 1982;81:278-84. [Crossref] [PubMed]
- Desai SJ, Mehta AC, VanderBrug Medendorp S, et al. Survival experience following Nd:YAG laser photoresection for principal bronchogenic carcinoma. Chest 1988;94:939-44. [Crossref] [PubMed]
- Cavaliere Southward, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. [Crossref] [PubMed]
- Wahidi MM, Herth FJ, Ernst A. Land of the fine art: interventional pulmonology. Chest 2007;131:261-74. [Crossref] [PubMed]
- Reddy C, Majid A, Michaud Grand, et al. Gas embolism post-obit bronchoscopic argon plasma coagulation: a case serial. Chest 2008;134:1066-9. [Crossref] [PubMed]
- Seaman JC, Musani AI. Endobronchial ablative therapies. Clin Chest Med 2013;34:417-25. [Crossref] [PubMed]
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Breast 2000;118:516-21. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Endobronchial electrosurgery: argon plasma coagulation and electrocautery. Semin Respir Crit Intendance Med 2004;25:367-74. [Crossref] [PubMed]
- Mahmood M, Wahidi MM. Ablative therapies for central airway obstruction. Semin Respir Crit Care Med 2014;35:681-92. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstacle. Chest 2001;119:781-seven. [Crossref] [PubMed]
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic Cryotherapy. Clinical Applications of the Cryoprobe, Cryospray, and Cryoadhesion. Ann Am Thorac Soc 2016;13:1405-15. [Crossref] [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Breast 1996;110:718-23. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999;20:123-38. [Crossref] [PubMed]
- Boujaoude Z, Young D, Lotano R, et al. Cryosurgery for the immediate treatment of astute primal airway obstacle. J Bronchology Interv Pulmonol 2013;20:45-vii. [Crossref] [PubMed]
- Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of cancerous stenosis. J Thorac Cardiovasc Surg 2010;139:997-chiliad. [Crossref] [PubMed]
- Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstacle. J Thorac Cardiovasc Surg 2004;127:1427-31. [Crossref] [PubMed]
- Inaty H, Folch Due east, Berger R, et al. Unimodality and Multimodality Cryodebridement for Airway Obstruction. A Single-Centre Experience with Safety and Efficacy. Ann Am Thorac Soc 2016;thirteen:856-61. [Crossref] [PubMed]
- Vishwanath G, Madan K, Bal A, et al. Rigid bronchoscopy and mechanical debulking in the management of central airway tumors: an Indian experience. J Bronchology Interv Pulmonol 2013;20:127-33. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC. Endoscopic relief of malignant airway obstruction. Ann Thorac Surg 1989;48:469-73. [Crossref] [PubMed]
- Hautmann H, Gamarra F, Pfeifer KJ, et al. Fiberoptic bronchoscopic balloon dilatation in malignant tracheobronchial illness: indications and results. Chest 2001;120:43-ix. [Crossref] [PubMed]
- McArdle JR, Gildea T, Mehta Air conditioning. Balloon Bronchoplasty: Its Indications, Benefits, and Complications. J Bronchology Interv Pulmonol 2005;12:123-seven.
- Kennedy MP, Morice RC, Jimenez CA, et al. Treatment of bronchial airway obstruction using a rotating tip microdebrider: a case report. J Cardiothorac Surg 2007;two:16. [Crossref] [PubMed]
- Lunn W, Bagherzadegan N, Munjampalli SK, et al. Initial Experience With a Rotating Airway Microdebrider. J Bronchology Interv Pulmonol 2008;fifteen:91-4.
- Casal RF, Iribarren J, Eapen G, et al. Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstacle. Respirology 2013;xviii:1011-five. [Crossref] [PubMed]
- Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg 2018;vii:273-83. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for cancerous and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72. [Crossref] [PubMed]
- Lunn W, Feller-Kopman D, Wahidi One thousand, et al. Endoscopic removal of metallic airway stents. Breast 2005;127:2106-12. [Crossref] [PubMed]
- Casal RF. Update in airway stents. Curr Opin Pulm Med 2010;16:321-eight. [Crossref] [PubMed]
- Furukawa K, Ishida J, Yamaguchi G, et al. The role of airway stent placement in the management of tracheobronchial stenosis caused by inoperable advanced lung cancer. Surg Today 2010;forty:315-20. [Crossref] [PubMed]
- Lee P, Kupeli E, Mehta AC. Airway Stents. Clin Chest Med 2010;31:141-l. [Crossref] [PubMed]
- Saji H, Furukawa One thousand, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with cardinal airway obstacle. Interact Cardiovasc Thorac Surg 2010;xi:425-8. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant key airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Mantovani G, Astara G, Manca G, et al. Endoscopic laser ablation equally palliative treatment of endobronchial, nonresectable, or recurrent lung cancer: assessment of its impact on quality of life. Clin Lung Cancer 2000;1:277-85. [Crossref] [PubMed]
- Mahmood K, Wahidi MM, Thomas S, et al. Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstacle. Respiration 2015;89:404-13. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Cancerous Central Airway Obstruction: Results of the AQuIRE Registry. Breast 2015;148:450-71. [Crossref] [PubMed]
doi: 10.21037/amj.2018.eleven.06
Cite this article as: Oberg C, Folch E, Santacruz JF. Management of cancerous airway obstruction. AME Med J 2018;3:115.
Source: https://amj.amegroups.com/article/view/4747/html
Post a Comment for "what is the procedure performed to gain access to the airway below a tracheal obstruction?"